The NSW Government is building a world-leading viral vector manufacturing facility, the first of its kind in Australia.
OVERVIEW
Viral vectors are a key component of many gene therapies. They are increasingly being used in therapeutic and preventative health interventions, including vaccines. NSW is a globally recognised leader in developing and delivering gene and cell therapies.
Establishing a local viral vector manufacturing industry will secure viral vector supply for Australian researchers and give patients access to groundbreaking therapies. This will improve health and help save lives.
The NSW Government has committed to expand and operate an advanced, licenced, clinical-grade viral vector manufacturing facility. The facility is located in the Westmead Health and Innovation District in western Sydney. It will have the production capacity to meet the growing demand for viral vectors for use in gene therapy research and clinical trials in Australia and internationally.
Investment in the facility will help create jobs and opportunities for local businesses, diversify local industries, and boost Australia’s global position as an advanced manufacturing industry.
The NSW Government has committed $134.5 million to establish the Facility and to manufacture viral vector products for research and clinical trials.
BENEFITS
The project will:
- Leverage Australia’s world leading cell and gene therapy research expertise
- Benefit academic and clinical communities that have made significant contributions to cell and gene therapies.
- Secure viral vector supply for Australian researchers and provide faster access for patients to advanced therapies through supporting local and clinical trials
- Create high-value, high-skilled jobs in the health and technology sectors
- Promote opportunities for local businesses, diversify local industries, and boost Australia’s global position within advanced manufacturing industry
- Provide an important educational platform for researchers, attracting students and high-performance research teams.
SCOPE
The project includes:
- Building a commercial grade viral vector manufacturing facility to service clinical trials (550L from 25L capacity)
- Design and fit out of facility located within Kids Research building and Innovation Centre and procurement of specialised equipment including laboratories, plant rooms, and services.
More information on this project can be found in the following factsheets:
Project overview
How do viral vectors save lives?
Benefits to research and education
Benefits to jobs and industry
Synergies between viral vector and mRNA technology
Frequently Asked Questions
Watch the video below to learn more about the viral vector manufacturing facility:
Contact us
For enquiries and more information about the viral vector manufacturing facility, please contact the Health Infrastructure Commercial Partnerships team:
Email:
HI-Commercial@health.nsw.gov.au
Phone: (02) 9978 5402
Please click on a section below for further information on:
Investment opportunities
The story so far
What are viral vectors?
Who can viral vectors help?
Benefits of viral vector manufacturing in Australia
NSW’s world-class health and innovation ecosystem
Westmead Health and Innovation District
Investment opportunities
The NSW Government is seeking industry partners to co-invest and help develop the viral vector manufacturing facility at the Westmead Health and Innovation District.
Investors have a unique opportunity to partner with the NSW Government in world-leading viral vector manufacturing, the first of its kind in Australia. The government’s support of modern manufacturing in this specialised domain provides a foundation on which investors can confidently develop and commercialise emerging technologies.
Partnering with the NSW government in the facility offers an unprecedented opportunity to invest early in a rapidly growing sector of medical health research.
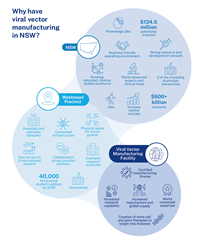
The viral vector manufacturing industry is directly driven by growth in the broader gene-modified cell therapy market, which is forecast to form a $120 billion market by 2035. Australia’s viral vector manufacturing market is expected to reach US$50 million by the end of 2026. As there isn’t a Good Manufacturing Practice (GMP) -licenced facility specifically dedicated to manufacturing clinical grade viral vectors in Australia, the facility is well-positioned to secure a majority market share.
Investing in the facility has the potential to seed new industries in cell and gene therapies, advanced manufacturing and biotechnology, and position Australia as a leader in viral vector manufacturing in the Asia-Pacific region.
For further information, download our Industry Prospectus or email:
HI-Commercial@health.nsw.gov.au.
 Back to the top
The story so far
The NSW Government is investing in a viral vector manufacturing facility that meets the demand for clinical-grade viral vectors for use in cell and gene therapy clinical research.
Back to the top
The story so far
The NSW Government is investing in a viral vector manufacturing facility that meets the demand for clinical-grade viral vectors for use in cell and gene therapy clinical research.
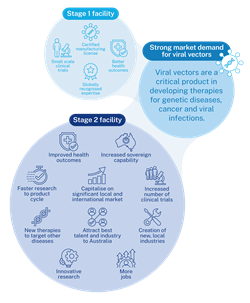
Existing viral vector infrastructure (stage 1) supports cell and gene technologies from discovery through to clinical trials for paediatric and adult patients. The expansion to a commercial-scale manufacturing facility (stage 2) will build further capability.
Stage 1 involved the construction of a 25-litre capacity pilot facility within the Westmead Health and Innovation District. Now operational, the pilot facility has established a workforce with specialist skills and is actively manufacturing a small quantity of viral vectors. This facility is in the process of obtaining Good Manufacturing Practice (GMP) certification, to enable the licensed production and sale of clinical-grade viral vectors.
Stage 2 will see the expansion of the pilot facility to a commercial-scale viral vector manufacturing facility with a 550-litre capacity. The stage 2 facility will further build capability while achieving financial sustainability by capturing the burgeoning national and international market for viral vectors. The commercial-scale facility will provide jobs for highly skilled staff, attract additional workers nationally and internationally and supply a larger and more varied customer base. In doing so, the stage 2 facility will support the development of intellectual property (IP) and attract further non-government investment.
Click graphic below to enlarge.
 Back to the top
What are viral vectors?
Back to the top
What are viral vectors?
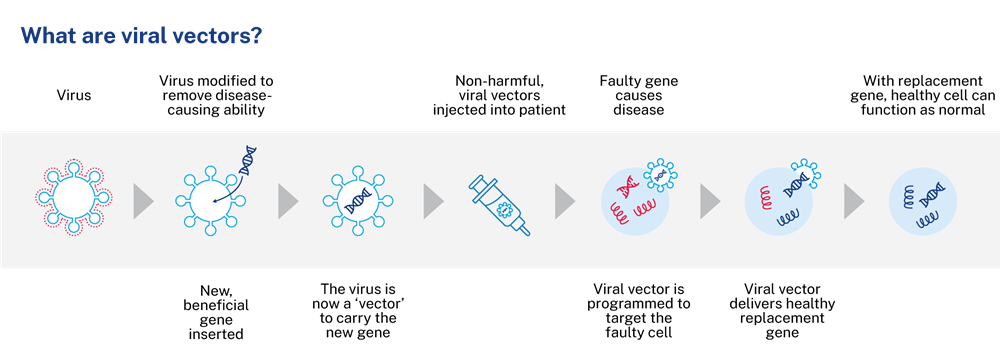
Viral vectors are an important tool used to make gene therapies. Gene therapies treat genetic diseases in a variety of ways; by replacing a faulty gene that is causing disease, adding genes to help the body fight or treat disease, or switching off genes that are causing disease.
Gene therapy relies on a carrier (vector) to deliver the beneficial genes into the body’s cells. Viruses are ideal vectors to carry information to our cells because they are naturally able to target specific cells.
When modified to remove their disease-causing ability, viruses can safely be used as vectors to insert cells with beneficial genes. By inserting working genes into the target cells, viral vectors provide the instructions the cell needs to function normally.
Viral vectors can be programmed to deliver genes to a wide range of cell types, and therefore can treat many diseases affecting a range of different organs. Viral vectors are the gene delivery vehicle used in approximately 70 per cent of gene therapies.
Click graphic below to enlarge.
 Back to the top
Who can viral vectors help?
Back to the top
Who can viral vectors help?
Viral vectors can help a broad range of patients. They can treat inherited genetic disorders, such as cystic fibrosis, spinal muscular atrophy (SMA) and blood disorders.
Patients with cancer can also benefit from viral vectors, as they can deliver genes that reprogram the patient’s immune system to fight cancerous cells. Viral vectors can also deliver other genes directly into cancer cells that instruct them to self-destruct.
Viral vectors also play an important role in vaccine development. Viral vector vaccines teach the body’s immune system how to respond to a foreign antigen. Recent viral vector vaccines have included the AstraZeneca COVID-19 vaccine.
Click the image of Jonathan below to read about the positive impact this technology has made in his life.
 Back to the top
Benefits of viral vector manufacturing in Australia
Back to the top
Benefits of viral vector manufacturing in Australia
Viral vectors are in demand. With rapid developments in our understanding of genetic diseases, the number of conditions that could be treated or cured using cell and gene therapies is growing. However, Australian researchers are waiting up to two years to source the viral vectors needed for cell and gene therapy clinical trials.
Local manufacturing of viral vectors will support the generation of treatments to improve Australian lives, stimulate the economy, and position Australia as a leader in health research, development and education.
Health benefits
Manufacturing viral vectors locally will provide Australian patients with faster access to innovative viral vector-based therapies to treat genetic diseases and cancer, and develop vaccines. This will result in more lives being saved and improved.
Economic benefits
Local manufacturing will stimulate the economy and create jobs supporting a wide range of medical, science, engineering and business skills. It will create a new global market for Australia and will help Australia retain high-value intellectual property.
Research and education benefits
Establishing local manufacturing infrastructure will accelerate national research by providing important medical tools to support clinical trials and research across Australia. It will also contribute to the education of the next generation of medical professionals.
 Back to the top
NSW’s world-class health and innovation ecosystem
NSW is at the forefront of international gene therapy research, with a world-class health and innovation ecosystem. It is well placed to support a viral vector manufacturing industry to create health, economic and research benefits for Australia.
Back to the top
NSW’s world-class health and innovation ecosystem
NSW is at the forefront of international gene therapy research, with a world-class health and innovation ecosystem. It is well placed to support a viral vector manufacturing industry to create health, economic and research benefits for Australia.
NSW is a globally recognised leader in developing and delivering gene and cell therapies. Australia’s first gene therapy clinical trial for a genetic disease was conducted in NSW in 2002. Since then, NSW Government-led initiatives in genomics and newborn screening have attracted additional clinical trials, leading to early access to cutting-edge cell and gene therapies for Australian patients.
Click graphic below to enlarge.
 Back to the top
Westmead Health and Innovation District
Back to the top
Westmead Health and Innovation District
The Westmead Health and Innovation District is one of the largest health, education, research and training precincts in Australia. It is an integrated medical and research hub that includes four hospitals, four world-leading medical research institutes, two multidisciplinary university campuses, including the largest clinical school in Australia, and the largest research-intensive pathology service in NSW.
The Westmead precinct provides a single point of entry to an ecosystem of globally recognised industry leaders, providing cell and gene therapy services from discovery through to clinical trials. Building in this precinct also offers convenient access to international collaborators via the Western Sydney Airport, which is currently under construction.
The viral vector manufacturing facility at the Westmead precinct will position NSW and Australia as a leader in future health research and innovation, promoting a culture of medical research and modern manufacturing in partnership with industry.
Back to the top
